Technology
Evaluation of tumor tissue is used for establishing a diagnosis as well as to determine status of therapeutically relevant biomarkers (gene variants, protein expression) which inform selection of targeted anti-cancer agents. However, tissue sample from a foundational biopsy captures the information at presentation and does not convey the evolution of molecular features which lead to (or following) treatment resistance and disease progression.
While targeted anti-cancer drugs are personalized and based on molecular profiling of tumors, the selection of chemotherapy agents (which are the mainstay of treatment regimens in several cancers) are not based on such molecular guidance. There are presently no means to identify patients in whom the cancer is likely to respond to chemotherapy agents (or in whom it may not).
Hence treatment failure leads to disease progression as well as to accumulated toxicities from successive inefficient treatments.
Patients with advanced or difficult to treat cancers, especially where the cancer is not responding to treatments, require intelligently designed strategies for selection of personalized regimens that evaluates and addresses the molecular and functional dynamics of the tumor.
An ideal strategy effectively targets vulnerabilities of the tumor and also pre-emptively circumvents known resistance mechanisms.
Selection of combination regimens with targeted and cytotoxic anticancer agents may act synergistically to prevent escape mechanisms of the malignancy and reduce rates of drug resistance.
Such a strategy can lead to more effective treatments which reduce the risks of treatment failure as well as the risks of toxicities associated with treatment failure.
About Exacta™
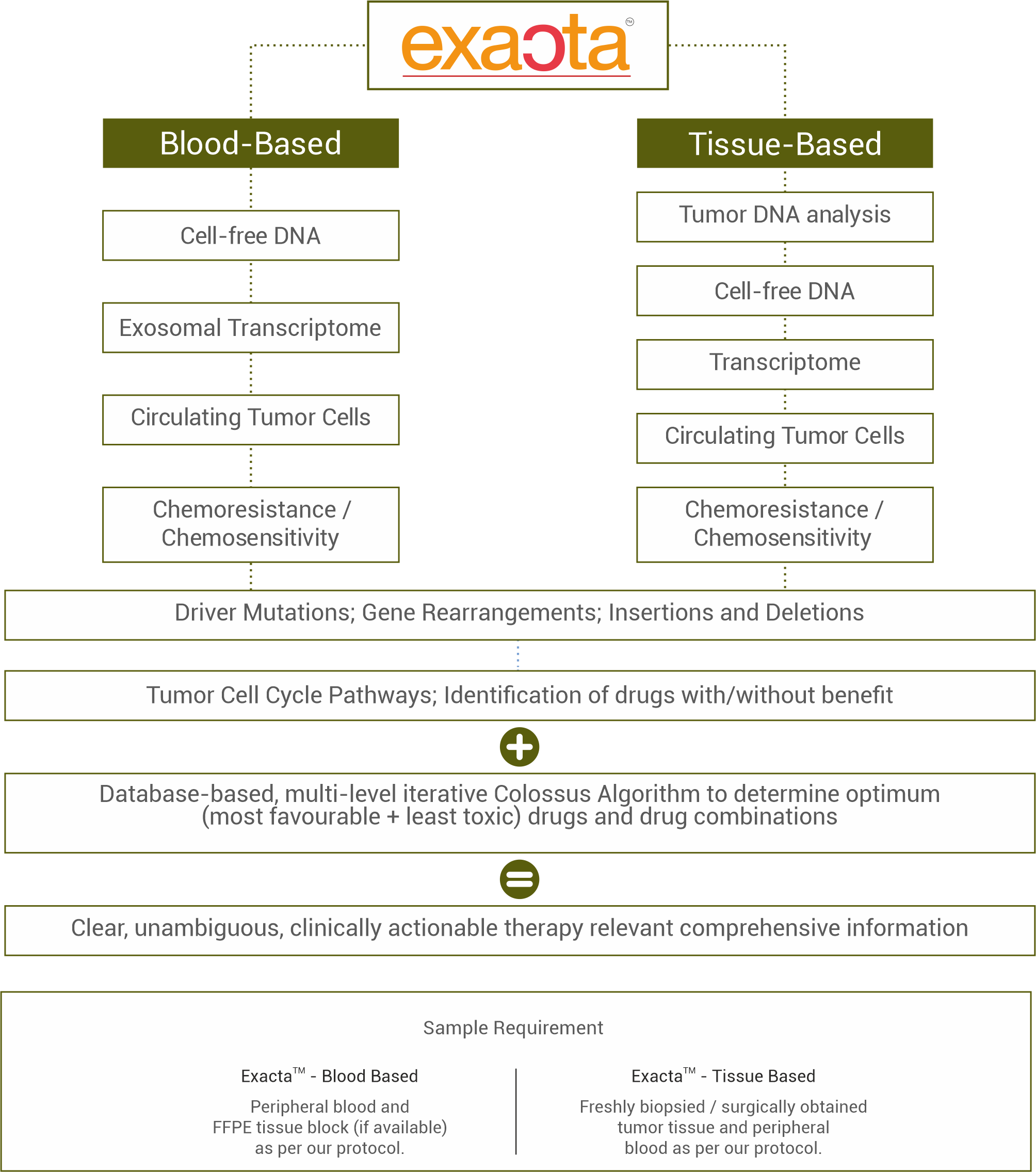
Exacta™ is an intensive and in-depth multi-analyte tumor profiling. It analyses millions of data points at the molecular and functional levels to reveal threats and vulnerabilities which enable optimal identification of targets for precision treatment selection.
Exacta™ evaluates:
- Gene variants such as point mutations (SNV, Single Nucleotide Variants), Insertions and Deletions, Translocations, Rearrangements and Fusions,
- Differentially up or down-regulated gene transcripts,
- Activated or suppressed significant molecular pathways,
- Abundance of protein targets,
- In vitro chemosensitivity / chemoresistance profiles of tumor cells
Exacta™ is agnostic to classical determinants such as primary organ, histological subtype, morphology (grade), aggressiveness and extent of disease.
Reliable Technology
Turn Around Time (TAT)
Typically, we are able to complete the test and analysis in 10 days and issue the final report and therapy recommendation within 12 days of the receipt of the sample(s) at our facility.
Clinical Performance
The clinical utility of Exacta™ for informing selection of patient-specific, safe and efficacious anti-cancer treatment regimens has been demonstrated in several clinical studies and case reports.
Exacta™ is available for all solid organ cancers.
Exacta™ can provide therapeutically relevant insights into the tumor. This helps physicians to make more effective life-saving clinical management decisions.